Abstract
Background: Janus kinases (JAK) family members (JAK1, JAK2, JAK3, TYK2) play an important role in tumorigenesis. JAK3 is involved in the signaling of a number of interleukins (IL) (including IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21), as well as the phosphorylation of the signal transducers and activators of transcriptions (STAT) and PI3K/AKT. JAK3 activating variants, including V722I, demonstrably induce cellular transformation in vitro. The prevalence of JAK3 variants/mutations in myeloid neoplasms, particularly in acute myeloid leukemia (AML), and their role in leukemogenesis, remains unclear.
Methods: We reviewed data for myeloid leukemia (AML, MDS and MPN) patients (pts) treated at MD Anderson Cancer Center between 2012 and 2017, whose marrow samples were tested for JAK3 variants/mutations by next generation sequencing. We evaluated the type of variants, the demographics, laboratory, treatment, and clinical information for pts with JAK3 variants. We performed an in vitro drug sensitivity assay using patient-derived xenograft leukemia cells from one patient with a JAK3 variant and using primary AML samples, to test for sensitivity to the JAK3 inhibitor tofacitinib.
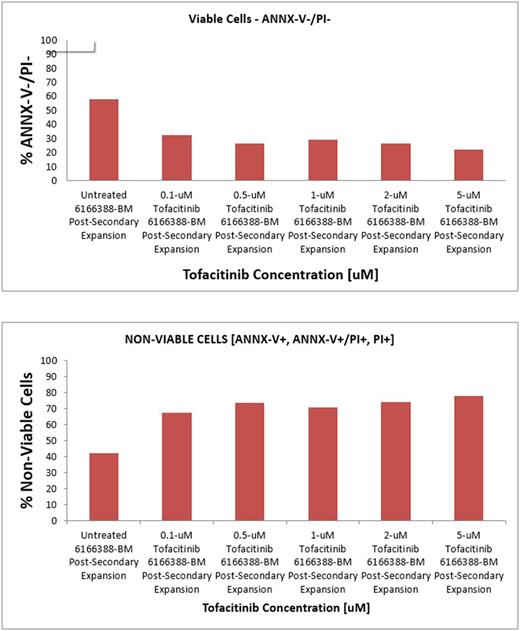
Results: A total of 35 leukemia pts with JAK3 variants were identified, and included: non-megakaryoblastic AML (n=31), MDS/MPN (n=2) and MDS (n=2). Four AML cases arose from prior MDS or MDS/MPN. All 4 non-AML pts had V722I variant, located in the pseudokinase domain. Of the 31 AML pts with JAK3 variants, 21 (68%) had V722I. Three (10%) other AML pts had P151R (FERM domain), 3 (10%) V718L (pseudokinase), 2 (6%) R925L (kinase), and 1 (3%) 940V (kinase); none of these variants has been previously described. A pt with T815M (between pseudokinase-kinase domains) was identified from outside genetic testing. The median allelic frequency for variants (where available) in AML pts was 53% (range, 41-88) and median marrow blasts was 45% (range 21-97). Overall, cytogenetics were complex in 19 (55%) pts and diploid in 11 (31%); 5 (14%) pts had miscellaneous clones. Concomitant somatic mutations most commonly occurred in IDH1/2 (n=12), TP53 (11), F LT3 (9), NPM1 (6), ASXL1 (5), RUNX1 (4), SRSF2 (4), and DNMT3A (3). Leukemia cells derived from a relapsed/refractory pt with JAK3 T815M variant had significant sensitivity to JAK3 inhibitor tofacitinib, with nearly 50% reduction in viability with 0.1uM drug concentration (Figure 1). Primary AML samples from 3 other pts showed no sensitivity to the drug.
Conclusions: We report the largest survey of JAK3 variants among adult myeloid leukemia pts, including novel variants. Given allelic frequencies, some JAK3 variants may represent germline polymorphisms. The activating V722I variant may have increased prevalence among AML pts. The sensitivity of JAK3 T815M AML cells treated with the FDA-approved JAK3 inhibitor tofacitinib, as well as the prevalence and location of JAK3 variants in myeloid malignancies, provide a basis for further investigation.
DiNardo: Novartis: Honoraria, Research Funding; Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding. Kantarjian: Bristol-Meyers Squibb: Research Funding; Novartis: Research Funding; Amgen: Research Funding; Pfizer: Research Funding; ARIAD: Research Funding; Delta-Fly Pharma: Research Funding. Verstovsek: Genentech: Research Funding; NS Pharma: Research Funding; Lilly Oncology: Research Funding; Galena BioPharma: Research Funding; Gilead: Research Funding; Promedior: Research Funding; Promedior: Research Funding; Gilead: Research Funding; CTI BioPharma Corp: Research Funding; Astrazeneca: Research Funding; Galena BioPharma: Research Funding; Genentech: Research Funding; Roche: Research Funding; Celgene: Research Funding; CTI BioPharma Corp: Research Funding; Blueprint Medicines Corp: Research Funding; Pfizer: Research Funding; Pfizer: Research Funding; NS Pharma: Research Funding; Blueprint Medicines Corp: Research Funding; Bristol Myers Squibb: Research Funding; Incyte: Research Funding; Seattle Genetics: Research Funding; Incyte: Research Funding; Seattle Genetics: Research Funding; Roche: Research Funding; Lilly Oncology: Research Funding; Astrazeneca: Research Funding; Bristol Myers Squibb: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal